Atoms To Mass In Grams Converter Kilograms
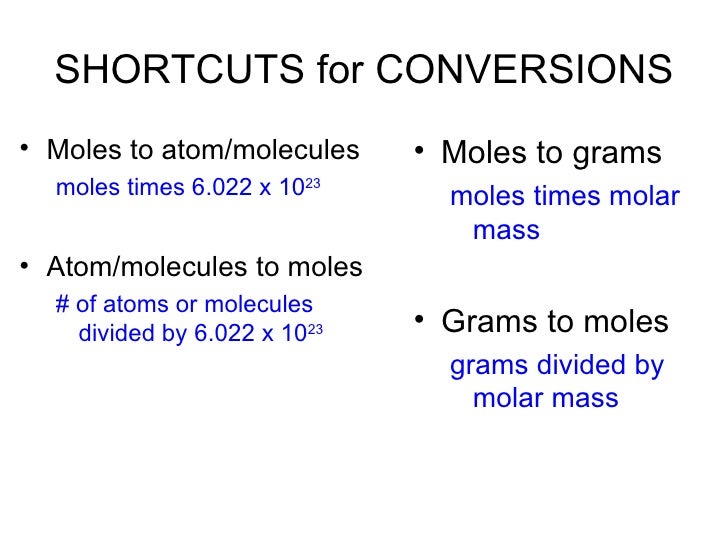
For chemistry you often need to convert moles to grams and grams to moles. There is a simple relation between these two:, where - mass of the substance in grams - quantity of the substance in moles - molar mass of the substance in grams/mole And the most difficult task here is finding out the molar mass of the substance. The molar mass is a physical property defined as the mass of a given substance (chemical element or chemical compound) divided by the amount of substance. The molar mass of atoms of an element is given by the standard relative atomic mass of the element multiplied by the molar mass constant, 1 × 10−3 kg/mol = 1 g/mol. The molar mass of a compound is given by the sum of the standard atomic weight (namely, the standard relative atomic mass) of the atoms which form the compound multiplied by the molar mass constant. Multiplying by the molar mass constant ensures that the calculation is dimensionally correct: standard relative atomic masses are dimensionless quantities (i.e., pure numbers) whereas molar masses have units (in this case, grams/mole). Happily, we already have the calculator, which calculates molar mass for given substance using handbook.
Atoms To Mass In Grams Converter Kilograms To Gallons
It is used in the calculator below to parse chemical compound formula and obtain molar mass. The calculator below calculates mass of the substance in grams or quantity of the substance in moles depending on user's input. It also displays molar mass of the chemical compound and details of its calculation just for reference. Note: Always use the upper case for the first character in the element name and the lower case for the second character as in periodic table. Compare: Co - cobalt and CO - carbon monoxide. Thus, Na3PO4 — correct notation, na3po4/NA3PO4 — incorrect notation.
Identify or measure the molecules of concern in amu. If you're doing this as a homework problem, the text will tell you how many atomic mass units the sample has. Multiply the amu measurement in Step 1 by 1.6605389 x 10^-27 to get the number of kilograms. This conversion number is in scientific notation. 10^-27 means that you move the decimal point in 1.6605389 to the left 27 positions.
How Many Grams In A Kilogram
This number is derived from Avagadro's number (see 'Tips'). Round to the number of significant figures of your Step 1 measure. For example, if you measure 101.1 amu, you’d round to 1.679 x 10^-25 kg so that both numbers have four significant figures. More explicitly, 101.1 amu x 1.6605389 x 10^-27 kg/amu = 1.011 x 100 x 1.6605389 x 10^-27 kg = 1.6605389 x (100 x 10^-27) kg = 1.679 x 10^-25 kg after rounding.